原標題:Bamberger苯并三嗪合成反應

苯胺重氮化后和丙酮酸腙反應得到重氮中間體后,苯并嗪在酸性條件下關環得到苯并三嗪的合成反應。此反應最早由 Eugen Bamberger在1892年報道。苯并嗪除了丙酮酸腙,合成苯甲醛苯腙也可以進行此反應。苯并嗪
合成反應機理
合成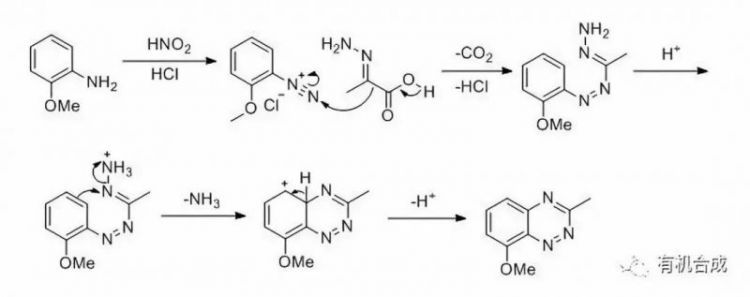
反應實例
合成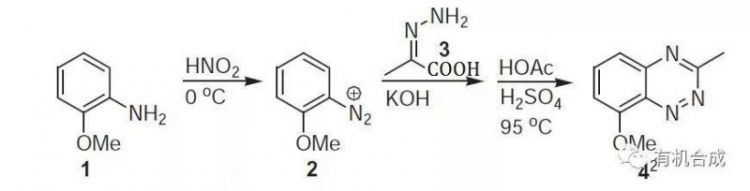
【Abramovitch RA, J Chem Soc., 1955, 2326】
合成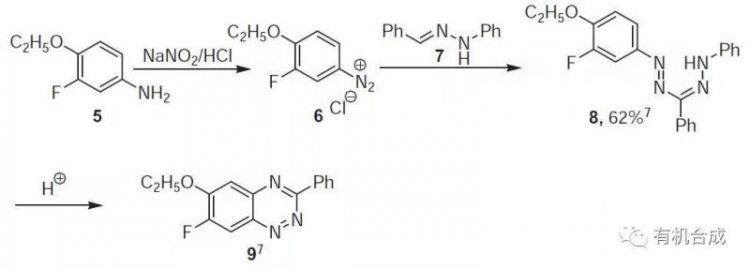
1-(4-Ethoxy-3-fluorophenyl)-3,5-diphenylformazan (8). To 2.9 g (0.02 mol) of 4-ethoxy-3-fluoroaniline 5 in 10 mL of 18% HCl acid was added dropwise at 0–5 ℃ a solution of 1.4 g (0.02 mol) of sodium nitrite in 5 mL of water and the mixture was stirred for 1 h at the same temp. This solution was added dropwise with stirring at 0 ℃ to of 3.2 g (0.016 mol) of 7 in 48 mL of pyr and the mixture was stirred for 3 h at 0–10 ℃. The cherry-red precipitate was filtered, washed, dried,and crystallized from EtOH to afford 8 (62%).
合成6-Ethoxy-7-fluoro-3-phenyl-1,2,4-benzotriazine (9).To formazan 8 (10 mmol) was added 7–10 mL of BF3/AcOH complex. The mixture was heated for 10 min at 100 ℃ (the color changed from violet to
合成dark-brown), then cooled to 0℃, and poured into ice-cold water (50 mL) to afford 9 on work up and crystallization (EtOH:CHCl3 2:1).
合成【Noronha G, Expert Opin Drug Dis., 2009, 4, 33】
合成相關文獻
合成1 Bamberger E Chem Ber 1892 25 3201
合成2 Abramovitch RA J Chem Soc 1955 2326
合成3R Abramovitch RA Chem Rev 1964 64 149
合成4 Katritzky AR Synth Comm 1997 27 3963
合成5 Khodja M Heteroatom Chem 2006 17 166
合成6 Aly AA ARKIVOC 2007 xvi 41
合成7 Noronha G Expert Opin Drug Dis 2009 4 33
合成編譯自:Organic Syntheses Based On Name Reactions, 3RdEd, A. Hassner, Page 26.


